About Us

Company Profile
Founded in 1981, China National Pharmaceutical Foreign Trade Corporation (Sinopharm Foreign Trade) is a wholly-owned subsidiary of China Sinopharm International Corporation (Sinopharm International) and a member company of China National Pharmaceutical Group Corporation (Sinopharm). As one of the first Chinese companies to engage in international businesses in the medicine and health sector, Sinopharm Foreign Trade has established unique strengths in international operation,medical institutions upgrade solutions, and supply chain services over the past 40 years or so.
MORE
Our History
“
1981
”
Founded as China Pharmaceutical Corporation for International Economic and Technological Cooperation (CPIC). As the window of the State Pharmaceutical Administration Committee (later NMPA) for international exchange, CPIC was mainly mandated to carry out economic and technological cooperation with foreign counterparts and facilitate new technology introduction and technological transformation of the pharmaceutical industry. It represented China's medical and pharmaceutical industry in cooperation with foreign multinational pharmaceutical companies to establish joint ventures in China.
Established the first Sino-foreign pharmaceutical joint venture, China Otsuka Pharma, with Otsuka Pharmaceutical Co., Ltd from Japan.
“
1982
”
Joined hands with Bristol-Myers Squibb of the United States to establish Shanghai Squibb Pharma, the first Sino-American joint venture in China in the pharmaceutical sector.
Joined hands with five Swedish companies to establish Sino-Swed Pharma, the first joint venture between China and Sweden.
“
1985
”
Partnered up with Warner-Lambert of the United States to establish Suzhou Capsugel Pharma.
Partnered up with Janssen, a Belgian pharmaceutical company that joined Johnson & Johnson of the United States, to establish Xian Janssen Pharma.
“
1988
”
Established Chongqing Glaxo Pharma with Glaxo.
Established Qingdao Huazhong Pharma with Kanebo of Japan.
Renamed as China National Pharmaceutical Foreign Trade Co. and started to engage in the import and export of medical and pharmaceutical technologies and products.
“
1991
”
Became one of the Top 500 companies by import and export aggregate in China by China's Ministry of Foreign Trade and Economic Cooperation.
“
1992
”
Started to use the English name SINOPHARM.
“
1994
”
Was designated by the State Council as one of the 100 enterprises for the modern enterprise system pilot program to kick off its reform towards a modernized enterprise.
Established the joint venture Zeneca Sinopharm Development Consulting Co. Ltd. with Zeneca Group PLC of the UK.
“
1998
”
Joined hands with China National Pharmaceutical Industry Co., Ltd., China National Medicines Corporation, and China National Medical Equipment Industry Corporation, all affiliated directly to the State Pharmaceutical Administration Committee to form China National Pharmaceutical Group Co., Ltd. (Sinopharm).
“
2002
”
Renamed as Sinopharm Foreign Trade Corporation (Sinopharm Foreign Trade).
“
2003
”
Set up a bonded warehouse for pharmaceutical products, i.e., the Sinopharm-FTZ Pharmaceutical Logistics Center.
Established Shanghai R&D Center in Shanghai Zhangjiang Hi-tech Park, marking a new step forward in increasing the technological level of pharmaceutical trade and integrating research, industrial development, trade, and services.
Sinopharm Foreign Trade was rated by Beijing Customs as an AA-level foreign trade enterprise, becoming one of the only 11 companies with the highest credit rating.
Passed the certification inspection of Good Supply Practice conducted by the Beijing Municipal Medical Products Administration, and became an pharmaceutical business in Beijing that passed the GSP certification carried out by provincial and municipal regulatory departments at an early stage.
“
2004
”
Obtained the ISO9001:2000 certificate from SGS.
“
2005
”
Signed an agreement with China National Corporation of Traditional & Herbal Medicine and Kanebo Ltd. of Japan to establish the joint venture HM Science Inc.
Obtained the ISO9001:2000 certificate for international labor cooperation and construction project contracting from China Quality Certification Center.
“
2006
”
Was included in the "red list" of import and export enterprises in 2005 released by China's General Administration of Customs together with 154 other enterprises.
The joint venture Vietnam-China Pharmaceutical Company Limited (now VCP) went into operation. It is one of the earliest injectable powder manufacturers in Vietnam to be certified to the Good Manufacturing Practice (GMP) of the WHO.
“
2008
”
Became the majority shareholder of Guangdong South Pharmaceutical Foreign Trade Company Limited.
“
2010
”
Joined hands with China National Service Corporation for Chinese Personnel Working Abroad and China National Corporation of Foreign Technical Cooperation in Medicine to establish China Sinopharm International Corporation.
China National Pharmaceutical Group (Vietnam) Pharmaceutical Co., Ltd was established in Hanoi.
“
2011
”
Sinopharm-FTZ Logistics Co., Ltd. was established as a joint venture with Development and Management Company Limited of Beijing Free Trade Zone.
“
2012
”
Became the majority shareholder of VCP (Vietnam).
“
2014
”
Sinopharm-FTZ Pharmaceutical Logistics Center went into operation.
“
2017
”
Completed corporate-style restructuring of central enterprises and renamed into Sinopharm Foreign Trade.
VCP built its TCM production line Phase Two and passed GMP certification in Vietnam.
“
2019
”
Introduced the Novartis third-party pharmaceutical logistics program to merge bonded and unbonded pharmaceutical warehousing services.
“
2020
”
Launched its first cooperation program with the WHO.
Honors & Qualifications
Membership of Industry Association
China Chamber of Commerce for Import and Export of Medicines and Health Products (executive member)
China Association of Medical Equipment (member)
China Chamber of Commerce of I/E of Foodstuffs, Native Produce and Animal Byproducts (member)
Beijing Pharmaceutical Association (member)
Beijing Customs Brokers Association (member)
The Japan China Medical Association (member)
China Association of Medical Equipment (member)
China Chamber of Commerce of I/E of Foodstuffs, Native Produce and Animal Byproducts (member)
Beijing Pharmaceutical Association (member)
Beijing Customs Brokers Association (member)
The Japan China Medical Association (member)
Food
distribution license
Radiation safety
license
Class II
medical devices Recordation certificate
medical device
Business license
ISO27001
Hazardous Chemicals Business permits
Drug
trade license
foreign aid materials
projects General contractor
AAA
corporate credit rating
National
integrity model for business
Shortlisted for emergency foreign aid materials projects
Class I Enterprise eligible for export tax refund for many years
ISO14001
AEO
advanced certified enterprise
ISO45001
ISO20000
ISO9001
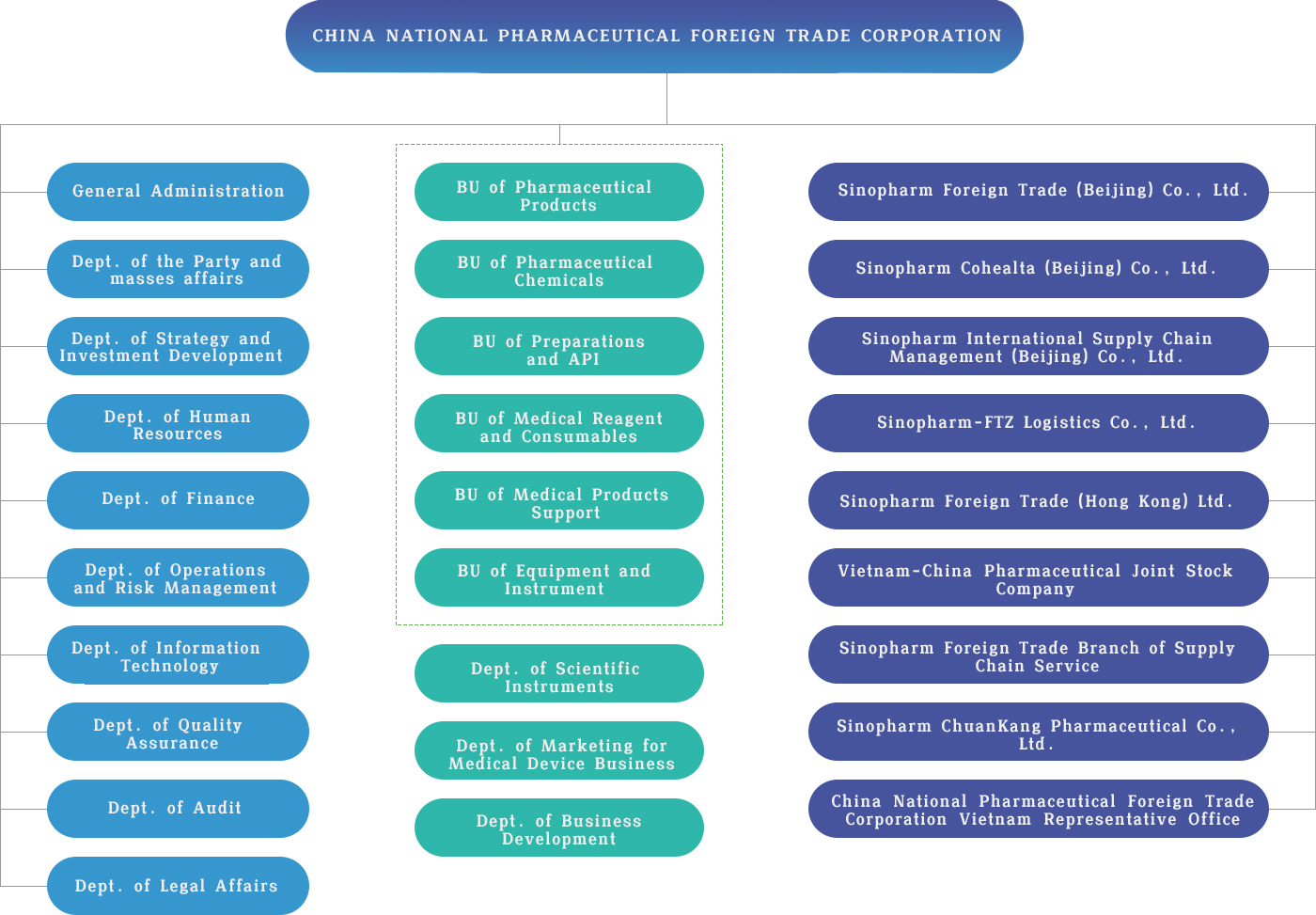
Organizational Structure